Insulin-Sensitizing Effects of Omega-3 Fatty Acids
Contents
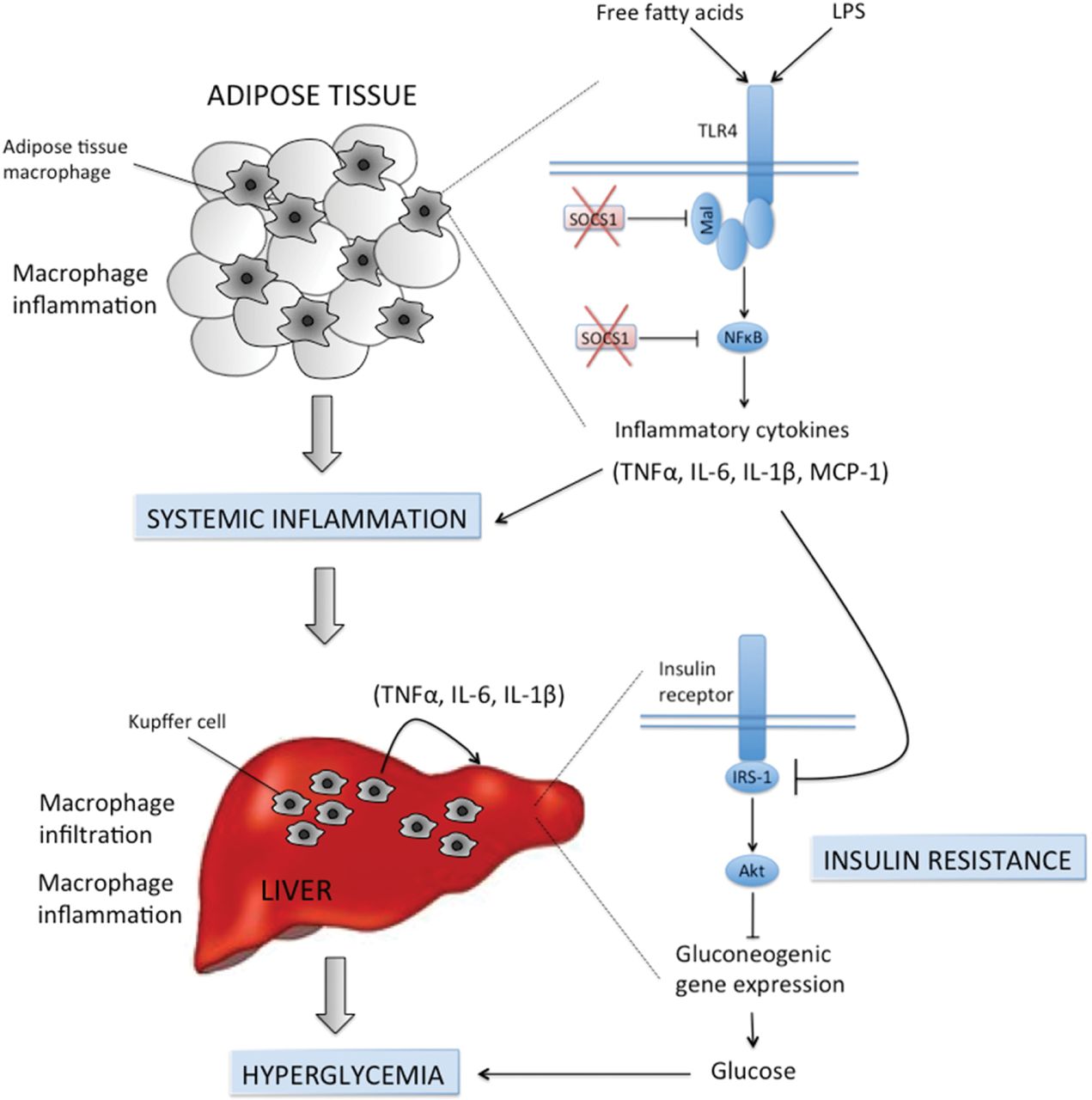
Overactivation of immune pathways in obesity is an important cause of insulin resistance
Overactivation of immune pathways in obesity is an important cause of insulin resistance and thus new approaches aimed to limit inflammation or its consequences may be effective for treating Type 2 diabetes. The SOCS (suppressors of cytokine signalling) are a family of proteins that play an essential role in mediating inflammatory responses in both immune cells and metabolic organs such as the liver, adipose tissue and skeletal muscle. In the present review we discuss the role of SOCS1 and SOCS3 in controlling immune cells such as macrophages and T-cells and the impact this can have on systemic inflammation and insulin resistance. We also dissect the mechanisms by which SOCS (1–7) regulate insulin signalling in different tissues including their impact on the insulin receptor and insulin receptor substrates. Lastly, we discuss the important findings from SOCS whole-body and tissue-specific null mice, which implicate an important role for these proteins in controlling insulin action and glucose homoeostasis in obesity.
http://www.biochemj.org/content/461/2/177.full
n-3 PUFA
EPA and DHA are very long chain polyunsaturated fatty acids (VLC n-3 PUFA) that incorporate into cell membranes following consumption of fatty fish, such as salmon, herring, mackerel, tuna, and sardines. Their biosynthesis is limited in the human body by relatively inefficient desaturase and elongase enzymes that convert ALA into VLC n-3 PUFA. Established properties of n-3 PUFA are their anti-inflammatory action and lowering effect on triglycerides [21], and they have been used as complimentary strategies in coronary heart disease [22,23] and retinopathy [24], while there is extensive research on improving cognitive disorders [25], telomeric aging [26], and cancer progression [27]. Much controversy still exists on their effect on glucose metabolism and insulin action. Mechanistic information about their action is still under investigation, however, their pleiotropic effects are purported to be related to their chemical structure. The long tail of double bonds confers some unique chemical and physical properties; they are extremely flexible molecules with rapid transitions between a large number of conformers, indicating a key property for entering into the binding pockets of proteins [28]. In addition, their cis bonds limit the ability of the fatty acids to be closely packed when incorporated into cell membranes, driving the formation of lipid domains, such as lipid rafts [29], which then enable or inhibit the interaction with signaling proteins and regulation of downstream pathways [29,30,31]. Thus n-3 PUFA are believed to affect membrane fluidity and also modulate expression of genes via regulation of transcription factors related to energy supply, cell cycle regulation, and cell differentiation [32].
Anti-Inflammatory Effects
Chronic macrophage-mediated inflammation is considered a hallmark of insulin resistance, and omega-3 fatty acids are purported to exhibit anti-inflammatory effects. A landmark study by Oh et al. reported that the G-protein-coupled receptor 120 (GPR120) expressed predominantly in mature adipocytes, macrophages, and hepatic stellate cells, functions as an omega-3 fatty acid receptor/sensor. By signaling through GPR 120, n-3 PUFA inhibit both TLR and TNF-α inflammatory signaling pathways and likely mediate M1–M2 macrophage polarization by decreasing the expression of inflammatory genes (IL-6, TNF-a, MCP-1, IL-1b, iNOS, CD11c) and increasing the expression of anti-inflammatory genes in adipose tissue (IL-10, MGL1, YM-1, Clec7a, MMR). GPR120 KO mice were glucose intolerant, hyperinsulinemic, and displayed skeletal muscle and hepatic insulin resistance, which was not ameliorated by n-3 PUFA supplementation. On the contrary, HFD-insulin-resistant WT mice supplemented with n-3 PUFA improved overall insulin sensitivity through mitigation of inflammation and increased adipose tissue glucose uptake [47]. Other studies also explored the prevention or reversal of IR in HFD-induced obese mice via modulation of adipose tissue inflammation, with a reported increase in anti-inflammatory cytokines (adiponectin) in plasma [51].
Hepatoprotection
Additional liver lipidomic profiling by Oh et al. revealed that n-3 PUFA ameliorated HFD-induced steatosis, supporting the view that omega-3 treatment can reverse non-alcoholic fatty liver disease (NAFLD) mediated in large part by GPR 120 [47]. Hepatoprotection and prevention of NAFLD, with repression of hepatic stellate cell activation and fibrogenesis in the liver, was also reported in mice fed HFD-enriched with fish oil for six weeks [39] and 12 weeks [52]. This was observed in parallel with a decrease in liver oxidative stress and was associated with improvements in HOMA-IR, adiponectin plasma levels, and overall insulin sensitivity. Liu X et al. also confirmed that EPA supplementation alone was efficacious in suppressing body fat accumulation and alleviated insulin resistance measured by OGTT in a HFD, HCD background. EPA alone also efficiently alleviated hepatic steatosis by modulating the suppression of adipocytokines (adiponectin) and inflammatory cytokines (TNFa, IL-6), and suppressed SREBP-1c-mediated lipogenesis while enhancing lipid β-oxidation (Liu, Xue et al. 2013). In line with these observations, a recent review by Delarue et al. concluded that LC-n-3 PUFA decrease liver steatosis, but do not reverse already established histologic features of non-alcoholic stehatohepatitis (NASH) [53].
A study by Poudyal et al. measured the independent effects of ALA, EPA, and DHA in high calorie-fed rats and all n-3 PUFA individually reduced inflammation in both heart and liver, as well as reducing cardiac fibrosis and hepatic steatosis. These effects were associated with complete suppression of stearoyl-CoA desaturase 1 (Scd-1) activity, a marker implicated in cardiovascular disease, insulin resistance, and obesity [37].
n-6:n-3 PUFA Ratio
A study demonstrating the anti-inflammatory properties of n-3 PUFA via suppression of toll-like receptor 4 (TLR4) activation in muscle reported improvements in inflammatory markers (TNFα, CRP, IL-6) and insulin resistance as measured from oral glucose and insulin tolerance tests (OGTT and ITT) [55]. These effects, however, were determined by a specific ratio of n-6:n-3 PUFA of 1:1, and were not replicated by a ratio of 4:1, suggesting that a balance among polyunsaturated fatty acids might be a more important contributor to metabolic health, rather than absolute levels of n-3 PUFA, as reported previously [56,57]. Other studies in non-rodent animals also attempted to delineate the metabolic importance of the n-6:n-3 PUFA ratio. Duan et al. fed pigs with either a 1:1, 2.5:1, 5:1, and 10:1 n-6:n-3 PUFA ratio and showed that the 5:1 ratio led to the highest growth performance, while the 1:1 diet led to increased muscle mass and lowest adipose tissue mass, with concomitant changes in inflammatory markers [58]. Although the metabolic significance of the n-6:n-3 PUFA ratio is still under investigation, considering the very high average ratio of the Western diet, 16–17:1, it is likely that an optimized n-6:n-3 PUFA ratio may exert beneficial effects on lipid metabolism, inflammation status, and other metabolic pathways.
n-3 PUFA Role in Aging
Aging is purported to be associated with a higher inflammatory status and insulin resistance. We, therefore, fed young and old mice with normal chow enriched with either EPA or DHA and performed large-scale proteomics and mRNA sequencing analysis from muscle tissue samples. Among the top metabolic pathways affected by EPA and DHA, was downregulation of the acute phase response signaling pathway, suggesting that n-3 PUFA ameliorated the inflammatory status in the muscle of old mice compared to young controls [32]. In addition, EPA supplementation enhanced muscle protein quality in the old mice by reducing carbamylation, a post-translational modification known to be driven by inflammation [32,59]. Further, we detected no differences in glucose tolerance or insulin sensitivity, indicating that n-3 PUFA might be beneficial only when a certain level of metabolic dysfunction is established, but not when insulin sensitivity is within a normal range. Aging is also associated with reduced mitochondrial function, which is implicated in the pathogenesis of insulin resistance [13]. Discussion of the effect of n-3 PUFA on mitochondrial function in the context of aging is reported under the section, “EPA and DHA Effect on Skeletal Muscle Metabolism”.
n-3 PUFA Effect and Inflammation
Inflammation is purported to be a leading mechanism in insulin resistance and metabolic disease. While most of the RCTs did not demonstrate a favorable effect of n-3 PUFA on insulin sensitivity, there are studies which reported improvement, as measured by HOMA-IR, and these studies had as a common denominator cohorts of participants with a high background inflammatory status. Browning et al., using a cross over study design, divided 30 overweight or obese women into tertiles of inflammatory status based on concentrations of sialic acid. Following 4.2 g/day of three-month n-3 PUFA supplementation and an oral glucose tolerance test, women in the top tertile for inflammation demonstrated improved insulin sensitivity, whereas those at the reference lowest tertile did not [85]. The women in the raised inflammatory status also had higher BMI and insulin resistance, as well as fibrinogen and CRP. However, the fact that the improvement in insulin sensitivity was not accompanied by significant changes in CRP, TNF-α, IL-6, α-1 anti-chymotrypsin, plasminogen activator inhibitor-1 (PAI-1), and α-1 acid glycoprotein (AGP), is suggestive that these plasma inflammatory markers are crude measurements of the n-3 PUFA anti-inflammatory effect or that the effect is mediated via other pathophysiological pathways. This corroborates the null findings from RCTs on relatively healthy adults with either moderate hypertriglyceridemia (Skulas-Ray, 2011 #5679) [95], or chronic lifestyle stress (Muldoon, 2016 #5670) [96] and low dietary EPA and DHA intake. Despite the beneficial effect on triglycerides, low, moderate, or pharmacological doses of n-3 PUFA had no effect on serum inflammatory markers (CRP and IL-6) versus a placebo.
n-3 PUFA and Energy-Restricted Diets
Improvement in insulin sensitivity as measured by HOMA-IR was also noted in conditions of hypocaloric diets [90,91], which suggested that fish oil exerts positive effects on fasting insulin, and this was reported to be independent of weight loss or changes in plasma triacylglycerol and adiponectin concentrations [90]. Of note, these beneficial effects were seen with relatively low doses of n-3 PUFA (Table 1). There still remains skepticism about whether the effects of omega-3 fatty acids were confounded by the robust insulin-sensitizing effects of caloric restriction [105], or whether there is a positive interaction of n-3 PUFA with dietary and exercise modifications of energy intake and expenditure.
In this regard, n-3 PUFA have also been studied for their efficacy in weight-loss interventions. This is particularly relevant because adiposity is a great determinant of insulin resistance [106]. Nevertheless, results have not been unanimous among human studies, with previous reports showing reduced body fat and increased resting fat oxidation in healthy adults [107], and reduced trunk fat and adipocyte diameter in type 2 diabetes patients, without changes in insulin sensitivity as measured by an insulin clamp [108]. When coupled with energy restricted diets, there was no effect of n-3 PUFA on body fat of young athletes [109] or overweight women [110], but there was a report of greater weight loss and waist circumference reduction in overweight men [111]. When fish oil was added to exercise regimens, there was an independent effect on body fat reduction in overweight and obese adults [112], but no effect in lean young male volunteers [113]. Also, in severely obese women, aerobic exercise plus a very low-calorie diet plus 2.8 g/day n-3 PUFA, led to a greater reduction in BMI and hip circumference compared to exercise plus diet plus placebo. Although these studies did not assess insulin sensitivity, they could indicate a potential beneficial role of n-3 PUFA via reducing adiposity. The modest reduction in body fat or adipocyte size in cohorts of obese adults is not in contrast with their aforementioned anabolic effect on skeletal muscle, since previous reports have been supportive of a tissue-specific action of n-3 PUFA.
n-3 PUFA in Type 2 Diabetes
A brief overview of randomized clinical trials in type 2 diabetes patients is summarized in Table 3. It has been long proposed that omega-3 fatty acids do not provide beneficial effects on the glycemic control of patients with established type 2 diabetes [2,117]. Two meta-analyses, including 18 and 23 RCTs respectively, with large numbers of participants, were concordant in outcomes, describing a decrease in triglycerides, a potential increase in LDL, and no effect on glycemic control or fasting insulin from n-3 PUFA supplementation [2,117].
In addition, meta-analyses of prospective studies had variable outcomes and reported no effect of n-3 PUFA on diabetes risk [126,127], beneficial effects in Asian populations [127,128,129], no associations among Europeans, and increased risk for incidence of diabetes in U.S. populations [128]. The observed differences between geographical regions could be a reflection of heterogeneous diets, including fish consumption, as well as racial and ethnic genetic differences. A more recent observational study by Lou DJ et al. [130] assessed the relationship of serum n-3 PUFA levels with IR and non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes, and reported that n-3 PUFA levels were significantly lower in T2DM and NAFLD and negatively correlated with HOMA-IR.
Among the most recent RCTs (Table 3), only one reported an improvement in HOMA-IR, with a concomitant decrease in NEFA following supplementation of 4 g/day for 2.5 months [118]. The majority of the studies which used the hyperinsulinemic-euglycemic clamp to assess insulin sensitivity, showed no beneficial effect of omega-3 fatty acids irrespective of duration and dosage [108,120,123,125]. There were, however, two randomized, double-blind, placebo-controlled studies by Mostad IL et al. which reported a moderate but significant deterioration of glycemia in subjects treated with fish oil. C-peptide responses also tended to be enhanced by fish oil, while an interesting effect was noticed in increased fat utilization compared to carbohydrate oxidation in the fasting state, following nine weeks of the intervention [121,122].
Skeletal Muscle Mitochondrial Function
Mitochondrial dysfunction is intertwined with the insulin resistance phenotype, as these organelles are responsible for lipid oxidation and for sustaining the metabolic demands of skeletal muscle. In this regard, we reported that fish oil increased expression of master transcriptional factors of mitochondrial biogenesis, including PGC1a and nuclear respiratory factor 1 (nrf1) [35]. Others also showed that omega-3 fatty acids are ligands for receptors that activate PGC1a [140], leading to an increased expression of PGC1a, mitochondrial transcription factor A (TFAM), cytochrome c oxidase, and mitochondrial membrane potential [141]. These studies indicate that dietary n-3 PUFA can prevent or reverse impairments in muscle mitochondria content or function. However, maximal mitochondrial oxidative capacity and phosphorylation efficiency did not change, pointing towards other mechanisms being associated with insulin signaling. This is consistent with reports in insulin-resistant humans, where oxidative capacity did not change after six months of EPA and DHA supplementation, measured in vitro from muscle biopsies and verified in vivo with magnetic resonance spectroscopy [76]. In light of these observations, Herbst et al. showed that EPA and DHA are incorporated into mitochondrial membranes and exert their effect by increasing mitochondrial sensitivity for ADP, and thus increasing submaximal, but not maximal, oxidative capacity [142]. Nevertheless, in the context of aging, where mitochondrial function is known to be impaired [13], EPA, but not DHA, partially improved mitochondrial oxidative capacity in old mice, without stimulating mitochondrial biogenesis or restoring age-related reductions in mitochondrial abundance. The effects were rather mediated by an improvement in mitochondrial protein quality by reducing deleterious post-translational modifications [32]. These findings would suggest that, depending on the underlying dysfunction (lipid overload, insulin resistance, aging) or severity, there could be differential effects of n-3 PUFA on skeletal muscle mitochondria function. In the context of aging, there are no available data, to date, on aged humans to support this assumption. However, we have unpublished data on elderly humans who were supplemented with 3.9 g/day of EPA/DHA for four months, which demonstrated that EPA/DHA could not reverse the age-related reduction in mitochondrial oxidative capacity, despite favorable effects on muscle protein homeostasis.
Conclusions
Despite promising strong outcomes in animal studies, there is no substantial cumulative evidence, to date, that VLC n-3 PUFA dietary supplementation can serve as a therapeutic strategy for insulin resistance in humans. Even less promising, are the data in regulating glycemic control in patients with type 2 diabetes. However, there is a strong indication that VLC n-3 PUFA may be used as preventive strategies based on their pleiotropic effects and their potential in regulating inflammation and innate immunity at the level of macrophages and the paracrine or endocrine function of cytokines. Although EPA and DHA have been mostly studied together, additional RCTs are needed to delineate their differential and independent effects elicited on muscle, liver, and adipose tissue with regards to insulin action. Further exploration and understanding of these mechanisms could be beneficial for long-term preventive strategies for chronic diseases and for promoting favorable outcomes in the acute clinical setting.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4924170/
A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats.
Dietary ratios of n-3/n-6 polyunsaturated fatty acids (PUFAs) have been implicated in controlling markers of metabolic disorders, including obesity, insulin resistance (IR), inflammation, and lipid profiles, which are also presumed to be partly related to type 2 diabetes mellitus (T2DM). However, molecular mechanisms of the different PUFAs related to metabolic disorders have not been systematically addressed. The present study aimed to investigate the impact of dietary n-3/n-6 PUFA ratios on obesity and IR and, further, to determine the underlying mechanisms. For 16 weeks, 32 SD male rats, randomly divided into four groups (n = 8 per group), received one of the following diets: normal chow, high saturated fatty acid (SFA), high n-3/n-6 PUFA ratio (1∶1, PUFA¹:¹), or low n-3/n-6 PUFA ratio (1∶4, PUFA¹:⁴). Following the experimental diet period, metabolic parameters related to obesity and IR were measured. Compared to SFA diet-fed rats, PUFA¹:¹ diet-fed rats exhibited decreased body and visceral fat weight, lowered blood lipids, and improved glucose tolerance and insulin sensitivity. Interestingly, these changes were accompanied with decreased expression levels of circulating pro-inflammatory cytokines, including tumor necrosis factor α, interleukin-6, and C-reactive protein. Moreover, the TLR4 protein and mRNA levels were markedly down-regulated by PUFA¹:¹ compared with SFA; however, PUFA¹:⁴ diet-fed rats failed to exhibit these changes. Cumulatively, our data highlight a role for a PUFA¹:¹ diet in the prevention of obesity and related metabolic disorders by suppressing the activation of TLR4, a critical modulator of pro-inflammatory cytokines.