Running on Empty
In 1971, a 27-year-old, 456-pound man went to the University of Dundee’s department of medicine in Scotland looking for help. Patient A.B., as doctors referred to him, needed to lose weight. His physicians recommended a short but drastic course of action: stop eating altogether. The patient responded so well to a brief stint without food that he decided to prolong the deprivation—for more than a year.
“[H]is fast was continued into what is presently the longest recorded fast (Guinness Book of Records, 1971),” the clinicians wrote in a 1973 case report, claiming A.B. suffered little or no untoward effects on his health.1 And at the end of his 382-day dietary abstinence, during which he had ingested only vitamin supplements, yeast, and noncaloric fluids, A.B. had lost a remarkable 276 pounds. When doctors checked back in on A.B. five years later, their patient reported gaining back only about 15 pounds.
Although aspects of this published report seem almost unbelievable, and the period of fasting is obviously extreme, the case highlights some of the metabolic dynamics that result when bodies are deprived of food. For example, when external calories stop fueling an animal’s metabolism, stores of triglycerides in fat cells are mobilized, and levels of ketones—chemicals that result from the burning of fat for fuel—rise. Decreases in body weight follow.
Scientists are further detailing both the underlying metabolic dynamics and interesting physiological phenomena aside from weight loss as they study less-extreme permutations of fasting in animal models and in humans. Data have recently emerged from research on several forms of so-called intermittent-fasting regimens, including alternate-day fasting, the so-called 5:2 diet, time-restricted feeding, and periodic fasting (see definitions). Although these regimens vary, they all involve a rhythmic disruption in the typical flow of calories into the metabolic machinery. “This is a simple intervention that has a profound impact,” says Satchidananda Panda, a researcher at the Salk Institute for Biological Studies in La Jolla, California, who studies the effects of time-restricted feeding.
As the body of scientific literature around fasting has grown, results have been cherry-picked and molded into fad diets that promise weight loss, increased energy, better sleep, and a variety of other benefits to human adherents—some with more evidential backing than others. As books of dubious scientific merit extolling the virtues of fasting fill the shelves, serious researchers continue to probe the genetic, immunologic, and metabolic dynamics that occur in fasting animals to separate hype from reality.
Contents
Adapting to lean times
For the majority of genus Homo’s more than 2 million–year evolution, hominins’ access to nutrients and calories was spotty, at best. Anyone who has hunted or gathered knows that success in either endeavor is not always guaranteed. But all of that began to change around 12,500 years ago, when H. sapiens invented agriculture, securing reasonably consistent streams of food—though the steadier diet constituted a narrower range of nutritional quality.
Perhaps our ancestors, and their digestive systems, evolved to endure periodic bouts of starvation, suggests University of Illinois at Chicago nutrition researcher Krista Varady. “I’m positive we didn’t have this constant flow or availability of food,” she says of preagricultural times. “Really it was based on the hunt. . . . And between those times, we were just eating a lot of low-calorie foliage. Whenever the big hunt would come in with an animal, then we’d have food for a couple of days, and then we wouldn’t.”
These oscillations between feast and famine may even have served as a selective pressure, tuning early human physiology to function optimally in an environment where resources were unpredictable. “Individuals whose brains and bodies and physical performance were optimal in a fasted state would be more likely to get food and compete with other individuals who were not able to function at quite as good a level,” says Mark Mattson, a neuroscientist who studies fasting diets at the National Institutes of Health’s National Institute on Aging (NIA). “So the assumption then is that we evolved probably most of our organ systems to be able to function optimally in intermittent fasting–type conditions.”
Oscillations between feast and famine may have served as a selective pressure, tuning early human physiology to function optimally in an environment where resources were unpredictable.
Panda, however, downplays the importance of feast and famine cycles in shaping our ancestors’ evolution. Instead, he contends that more-contemporary disruptions to the body’s many circadian clocks—which sense dark/light and feast/famine cycles, among other oscillations—are at the root of modern humanity’s mounting struggles with obesity and metabolic disease. “Undeniably, the amount of food available to us has increased, but another thing that has happened is the invention of [electric] light,” he says. “When there was darkness in the evening, of course people didn’t have much to do. . . . The light enables us to stay awake later in the night. And now we have plenty of food, so we tend to eat.”
Even with this disagreement concerning evolutionary vs. industrial justification for designing modern-day fasting diets, results from studies in both animal models and humans point to distinct benefits of withholding food in one temporal pattern or another. In recent years, scientists have learned that fasting might trigger not only weight loss and life-span extension—benefits that have long been linked to caloric restriction—but also boost the performance of the brain, the immune system, and organs central to metabolism, such as the liver and pancreas. Fasting, some researchers claim, can even alter the course of some diseases, from cancer and multiple sclerosis to diabetes and Alzheimer’s.
The fasting signal starts
| A BODY WITHOUT FOOD Mounting evidence suggests that intermittent fasting causes significant changes to various organs and tissue types. The fasting signal likely starts in the liver, the body’s central command for metabolism. But through changes in gene expression and alterations in complex enzymatic pathways, the effects of food deprivation spread throughout the body, from the brain and visceral fat to the muscles and more. |
|
 |
Liver Fasting and time-restricted feeding increases insulin sensitivity, decreases insulin resistance, and lowers blood glucose levels. With prolonged periods of fasting, the liver’s glycogen stores become depleted, and visceral fat is tapped as an energy source, which releases ketones that can be metabolized by neurons and muscle cells. |
 |
Immune System Periodic fasting reprograms T-cell populations, tamping down autoimmunity and rescuing immunosenescence. A lack of incoming calories appears to prune away autoimmune T cells, and with refeeding, hematopoietic stem cells are activated to replace T cells, lymphocytes, and other white blood cells. Several fasting studies have also pointed to a decrease in inflammatory cytokines. |
 |
Heart Because triglycerides become mobilized for energy in the absence of incoming dietary calories, blood lipid levels tend to go down in a fasting body. Researchers have also seen decreases in blood pressure in fasting animals. In some animal studies of fasting, investigators have recorded decreases in cholesterol. |
 |
Brain Intermittent fasting has improved memory, learning, and neurogenesis in rodents, and has been shown to repair some neurons in mouse models of ischemic stroke. |
 |
Cancer By making tumor cells more susceptible to chemotherapeutic agents while protecting healthy cells from the treatment’s toxicity, intermittent fasting is showing promise in slowing the progression of breast cancers and melanoma in mice. |
In seeking to understand how a body reacts to a dearth of incoming calories, it makes sense to start in the liver, an organ crucial for the processing of nutrients flowing into a body’s metabolic machinery. Panda has documented profound alterations in gene expression in the liver cells of animals subjected to conditions of fasting. In a 2009 PNAS study, he and colleagues found that when they withheld food from mice for 24 hours, 90 percent of the genes expressed in a cyclical, circadian clock–driven fashion by liver cells ceased to oscillate.2 “That means most of the cycling comes from these instructions from food,” he says.
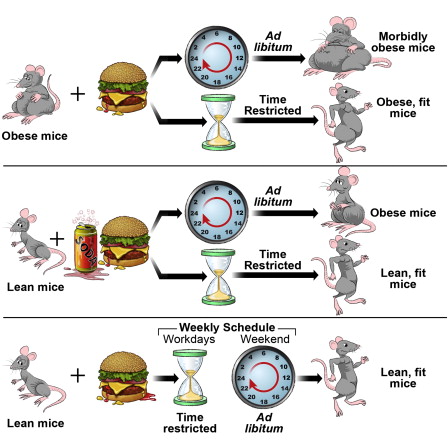
Conversely, Panda’s lab later showed that feeding mice high-fat diets with food accessible 24 hours a day similarly breaks circadian clocks in the liver, resulting in an enhanced propensity for obesity and its attendant maladies. But making that same high-fat diet—and the same number of calories—accessible to the animals for only eight hours per day during their normal wake cycles protected the mice from the development of a host of metabolic diseases.3 “The mice were completely protected from diabetes, cardiovascular disease, high cholesterol, fatty liver disease, these diseases that affect most of our elderly,” he says.
See “Out of Sync”
Altering the feeding window to regimens of 8, 9, or 12 hours didn’t make much of a difference. “In most cases we saw protection from all of these diseases, and if the mice were fat enough initially, they lost 20 percent of their body weight and many of their diseases reversed,” Panda says, adding that they also tried a 15-hour feeding window, which did not offer too much disease protection.
Panda says that the mechanisms underlying the physiological responses to fasting likely involve a number of metabolic pathways commonly studied by nutrition scientists, including mTOR, insulin, AMP kinase, and protein kinase A (PKA). These same pathways turned up recently in a study by the University of Southern California’s Valter Longo, who examined the consequences of fasting on another crucial metabolic organ, the pancreas. He and collaborators showed that periodic fasting using a fasting-mimicking diet (FMD)—which is low in calories, carbohydrates, and proteins but contains ample fatty acids to push the body into a fasting state without a complete cessation of incoming energy—promoted the functional repair of damaged pancreatic cells in mouse models of type 1 and type 2 diabetes by reducing PKA and mTOR activity, while increasing insulin production.4 (Longo started a company, L-Nutra, which sells FMD diets; he donates his profits to charity.)
The hungry brain
Mattson, who studies the brains of mice, rats, and humans in his lab at the NIA, has identified neurological benefits of fasting and suggests that the adaptive stress response may be at their foundation. The gist of this response is that, under unfavorable environmental conditions such as toxin exposure, extreme heat, or a lack of readily available fuel, cells in an animal’s body alter gene expression to crank out proteins that play protective roles. These proteins make cells more resistant to the stressor through enhancing DNA repair or by stabilizing proteins’ 3-D conformations, among other mechanisms. In the brain, the adaptive stress response can strengthen neuronal networks and enhance neural plasticity.
In recent years, Mattson and colleagues have homed in on brain-derived neurotrophic factor (BDNF) as a key mediator of neurological and other fasting responses. Their research has revealed that this growth factor causes an increase in the number of mitochondria in hippocampal neurons, perhaps explaining why rats on fasting cycles not only gain less weight than their continuously fed counterparts, but perform better at learning and memory challenges.5 Intermittent fasting appears to calm inflammation and rescue cognitive function in the animals’ brains by knocking down the quantity of proinflammatory cytokines, elevating levels of BDNF, and activating the transcription factor known as NF-κB, which is involved in adaptive stress responses.6 In addition, BDNF activates PGC-1α, which regulates the production of new mitochondria, dendritic spines, and synapses in the hippocampus.7
Last year, Mattson and collaborators further elucidated the complex molecular mechanics that link intermittent fasting with improved brain function and neuroprotection. The researchers demonstrated that the mitochondrial deacetylase SIRT3 is central to mediating adaptive stress responses in mice that are exposed to prolonged exercise or periods of fasting. Under these conditions, SIRT3 levels in hippocampal neurons ramp up, improving neural network function and boosting mitochondria production in the hippocampus.8 “There’s a story emerging whereby exercise . . . and intermittent fasting as well, upregulates SIRT3 and protects mitochondria against stress,” says Mattson. “And that seems important for exercise and intermittent fasting to allow the cells to grow and new synapses to form.”
Rebuilding defenses
Fasting that involves longer periods of food deprivation can cause changes to the immune system and the hematopoietic stem cells that support it. Longo studies periodic fasting, less frequent but longer bouts of severe calorie restriction, and is finding that the routine can reshape immune cell populations in the body. For most of his studies, Longo uses the fasting-mimicking diet (FMD).
Using a periodic three-day FMD regimen for 30 days in a mouse model of multiple sclerosis, Longo and colleagues showed that the fast-and-feed cycles pruned away populations of autoimmune T cells, replacing them with immune cells that were no longer bent on attacking neural tissue. Oligodendrocyte precursor cells regenerated and remyelinated axons, and the clinical severity of the autoimmune disorder declined.9 “One in five of the mice went to back to no symptoms at all. One in two of the mice went down to very low levels of the symptoms,” Longo says.
Fasting that involves longer periods of food deprivation can cause changes to the immune system and the hematopoietic stem cells that support it.
“The real benefit that we’ve shown in a number of papers, and we have several coming out very soon, is about killing damaged cells and then turning on stem cells,” Longo adds. “And then in the refeeding period, [stem cells are] replacing the dead cells with newly generated cells. I think that is where the real benefit is.”
Healing, fast
Much of the basic biological work on fasting diets is done in animal models of disease, demonstrating improvements in or protection against symptoms of Alzheimer’s, multiple sclerosis, cardiovascular disease, diabetes, and other metabolic disorders. Longo has also turned his sights on cancer. He and his team showed in mouse models of breast cancer and melanoma that periodic fasting with a four-day FMD for about a month, combined with chemotherapy, delayed tumor progression by sensitizing the malignant cells to chemotherapy, while protecting normal cells from the toxins. This effect, they found, was mainly mediated by increases in the levels of lymphoid progenitor cells and tumor-killing lymphocytes.10 “On one side, the fasting promotes the regeneration of the immune cells and they’re more aggressive, but more importantly, it’s acting on the breast cancer cells and also the melanoma cells to make them more exposed in a way to the T cells,” Longo says.
Mattson, too, has tested the effects of fasting on specific disorders. In 2014, he participated in research that illustrated the contribution of time-restricted feeding—in this case, feeding mice in an eight-hour window every day for four months—to tamping down inflammatory responses after ischemic stroke was induced in the animals. The study implicated some of the same neuronal signaling pathways—NF-κB and MAPK, for example—in the fasting regimen’s ability to calm the cytokine storm that attacks brain tissue after stroke.11 “There’s some evidence coming out that intermittent fasting after stroke in animals can enhance their recovery,” Mattson says.
Longo, working as the director of USC’s Longevity Institute, is now directing his questions about fasting and cancer progression to humans. “Now we have about 400 patients that are enrolled or will be enrolled in multiple clinical trials that we’re running with the fasting-mimicking diet,” he says.
The fasting fad
As often happens with nutrition research, results from fasting research have been borrowed and packaged into ill-informed fad diets. Panda’s research formed the basis of one such product, branded as The 8-Hour Diet. The book, cowritten by an editor at Men’s Health, which covered Panda’s research when it came out in 2012, claimed (right on the cover) that dieters who restricted their food consumption to an eight-hour window every day could “watch the pounds disappear without watching what you eat!” This idea, says Panda—who was not involved in producing the book—was unsupported by his research as of 2012. “We never claimed that we could make fat mice lean with the eight-hour diet,” he says. “In our first paper, we just prevented obesity from happening.”
Nevertheless, Panda has gone on to show that there may be some substance to the weight-loss hype. In addition to his experiments in mice, Panda’s team found that flies, too, experienced benefits from restricting their feeding windows to a confined period each day. For the insects, a 12-hour feeding window proved to be best, with flies eating for only half a day experiencing better-quality sleep, less weight gain, and a deceleration of cardiac aging compared to their counterparts who were fed the same number of calories, but in food administered throughout the day.12
Extending the research into humans, Panda’s lab recently published a study of eight otherwise healthy people with body mass indices between 25 and 30 (in the “overweight” range, according to the National Health and Nutrition Examination Survey). When the study subjects ate all of their food for the day in 10 hours, instead of 14 or 15 (as they tended to do before the study), they lost an average of 4 percent of their body weight and reported having significantly more energy and more satisfying sleep over the 16-week trial period, with the benefits sticking around for up to a year.13
Most intermittent-fasting regimens, including alternate-day fasting and the 5:2 diet (fasting for two days each week), have shown promise in helping people and experimental animals shed pounds. Unlike time-restricted feeding, however, these approaches are really caloric restriction in disguise. By severely limiting calories every other day or every third day, dieters reduce their caloric intake over the course of a week or month, without the annoyance of constantly counting calories or restricting portions at every single meal. “That’s what intermittent fasting is, it’s just tricking your mind and body into eating less, and because you’re losing weight, you’re getting all these metabolic benefits,” says Varady, who studies alternate-day fasting and wrote a book, The Every-Other-Day Diet, as a summary of her research and a recipe guide. Last month, she and colleagues published results of a clinical trial comparing obese, but otherwise healthy, people practicing alternate-day fasting to a similar group restricting calories on a daily basis. Both cohorts lost roughly the same amount of weight over the course of one year, and both diet regimes had similar noncompliance rates.14
Intermittent fasting, whether you do two days a week, every-other-day fasting—some of the things that are being used the most—they’re extremely difficult for people to do.—Valter Longo
University of Southern California
Varady notes that most of the trial subjects on whom she’s tested alternate-day fasting tend to report a certain amount of ease with complying with the diet, and she’s found that they tend not to compensate for fasting days by consuming drastically more calories on feast days. “It’s almost like they can’t binge on the feast day. They typically only consume about 15 percent more energy than they normally do,” she says.
Richard Bloomer, a University of Memphis nutritional scientist who studies dietary restriction in animals and humans, says that long-term compliance is still an unanswered question about the practicality of intermittent-fasting diets. “The compliance issue is crucially important. Which of these plans, regardless of the outcomes, allow people to maintain them longer term?” he asks. “That’s probably one of the more important things to study as opposed to simply the mechanisms as to why things are occurring.”
And for some researchers, intermittent-fasting plans, such as alternate-day fasting and the 5:2 diet, are doomed to a fate similar to that of caloric restriction. “Intermittent fasting, whether you do two days a week, every-other-day fasting—some of the things that are being used the most—they’re extremely difficult for people to do,” says Longo. “There’s very little data, actually, long-term, and my guess is that, just like caloric restriction, you’re going to see the good and the bad, and it’s potentially going to be a failed operation.”
“I think intermittent fasting right now is probably another nutritional fad,” says Varady. “I’ve been in nutrition research not for a super long time but for about 15 years or so, and I just noticed every 10 or 15 years there’s something new that comes up.”
Nevertheless, she adds, “I still think that it can really help people out, and I think people who are able to stick to it really reap a lot of metabolic benefits.”
| TYPES OF INTERMITTENT FASTING
All fasts are not created equal. Researchers are studying several different permutations of intermittent fasting, which all provide ample drinking water to subjects, in laboratory animals and in humans. Alternate-day fasting: Subjects eat every other day. For humans, noneating days typically consist of one small meal of around 500 calories, amounting to a dietary energy reduction of approximately 65 percent to 80 percent. 5:2 diet: A person eats five days of the week and abstains from eating the other two (for example eating on Monday, Wednesday, Friday, Saturday, and Sunday and fasting on Tuesday and Thursday)—save for one small, 500-calorie meal on fasting days (cutting dietary energy by about 65 percent to 80 percent on those days). Periodic fasting: This fast is undertaken anywhere from once a month to once a year. For a period of at least five days, food is avoided or subjects eat a modified “fasting-mimicking diet” that steps down energy intake over the fasting period and is low in carbohydrates, proteins, and calories. Time-restricted feeding: Calories are not restricted, and dietary composition is not altered. But eating is confined to a window of typically 8, 10, or 12 hours per day. |
References:
- W.K. Stewart, L.W. Fleming, “Features of a successful therapeutic fast of 382 days’ duration,” Postgrad Med J, 49:203-9, 1973.
- C. Vollmers et al., “Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression,” PNAS, 106:21453-58, 2009.
- M. Hatori et al., “Time restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high fat diet,” Cell Metab, 15:848-60, 2012.
- C.W. Cheng et al., “Fasting-mimicking diet promotes ngn3-driven β-cell regeneration to reverse diabetes,” Cell, 168:775-88, 2017.
- K. Marosi, M.P. Mattson, “BDNF mediates adaptive brain and body responses to energetic challenges,” Trends Endocrinol Metab, 25:89-98, 2014.
- A.R. Vasconcelos et al., “Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment,” J Neuroinflamm, 11:85, 2014.
- Cheng, et al., “Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines,” Nat Commun, 3:doi:10.1038/ncomms2238, 2012.
- A. Cheng, et al., “Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise, and metabolic and excitatory challenges,” Cell Metab, 23:128-42, 2016.
- I.Y. Choi et al., “Diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms,” Cell Rep, 15:2136-46, 2016.
- S. Di Biase et al., “Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity,” Cancer Cell, 30:136-46, 2016.
- D.Y. Fann, et al., “Intermittent fasting attenuates inflammasome activity in ischemic stroke,” Exp Neurol, 257:114-19, 2014.
- S. Gill et al., “Time-restricted feeding attenuates age-related cardiac decline in Drosophila,” Science, 347:1265-69, 2015.
- S. Gill, S. Panda, “A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits,” Cell Metab, 22:789-98, 2015.
- J.F. Trepanowski et al., “Effect of alternate-day fasting on weight loss, weight Maintenance, and cardioprotection among metabolically healthy obese adults: A randomized clinical trial,” JAMA Intern Med, doi:10.1001/jamainternmed.2017.0936, 2017.