Diet and Inflammation. Part 4
But what about the initiation of the inflammatory signal in the first place? That often turns out to be due to a widespread deficiency in the amino acid glycine, which I described in an earlier post. Glycine acts as a sort of “cellular voltage regulator”, preventing the inappropriate initiation of inflammation in response to cellular injury. (Inflammation is really only appropriate when there is infection taking place.)
But if glycine is the most abundant amino acid in the body, and it is nonessential—i.e., the human body can make it from scratch—why should anyone be deficient, especially nowadays, when the dietary intake of protein is usually so high?
As Matt Stone often says, it is really a matter of context; a matter of balance, rather than a matter of absolutes. Glycine is so abundant that it comprises about 22% by weight of the most abundant protein in the body. That protein would be collagen, the tough, extracellular fibrous protein that makes up the bones, cartilage and all the connective tissues. Hence, the collagen—and therefore most of the glycine—is the part of the meat, fish and poultry that we usually throw away. Bone broth is one way to recover it in the diet, and when the collagen is boiled out of the bones and purified, it is called gelatin.
But even though we discard most of the glycine from our animal flesh foods, we are still taking some in with our muscle meats, so that still doesn’t explain a widespread glycine deficiency. But in fact, the consumption of muscle meats actually exacerbates the deficiency because of the amino acid content of the muscle meats. Specifically, muscle meats are very rich in the essential amino acid methionine, and it is an understanding of the intimate relationship between methionine and glycine that provides the answer to this question of balance.
A problem with traditional nutritional thinking on amino acids is the rigid classification of those which are essential, as opposed to those which are non-essential; in particular, a disproportionate interest in the former v. the latter. Methionine, in particular, has long been known to play key roles in metabolism independent of its role as a constituent of proteins. Specifically, methionine—when activated to form S-adenosylmethionine (SAMe), is the universal methyl group donor, which adds a one-carbon methyl group (CH3 group) to a variety of important metabolic intermediates, including DNA bases and neurotransmitters.
So fundamental is this role of methylation, that the body (essentially, the liver) has numerous pathways of conserving, recycling, and salvaging methionine, so that it can withstand long periods of reduced methionine intake. So intense has been the emphasis on the essentiality of methionine, that methionine deficiencies have been hypothesized to underlie a number of pathologies, including cancer. But nothing could be further from the truth. In fact, typical Westerners consume about 10 times more methionine than is needed to support good health. (We really only need about 300 – 500 mg per day of methionine: more like the daily requirement for a B vitamin than a bulk nutrient!) Over 20 years’ worth of animal research has shown that laboratory animals (rats and mice) live substantially longer and healthier lives if their normal methionine intake is reduced by 80%. Moreover, it is now understood that metabolically, far from salvaging, recycling and regenerating methionine, the body switches gears and gets rid of most of the methionine absorbed from a typical high-protein meal.
What’s that got to do with glycine? The answer is remarkably simple: There is only one metabolic pathway that exists in the human body to get rid of excess methionine. That pathway—via the enzyme glycine-N-methyltransferase (GNMT)—uses up glycine in the process.
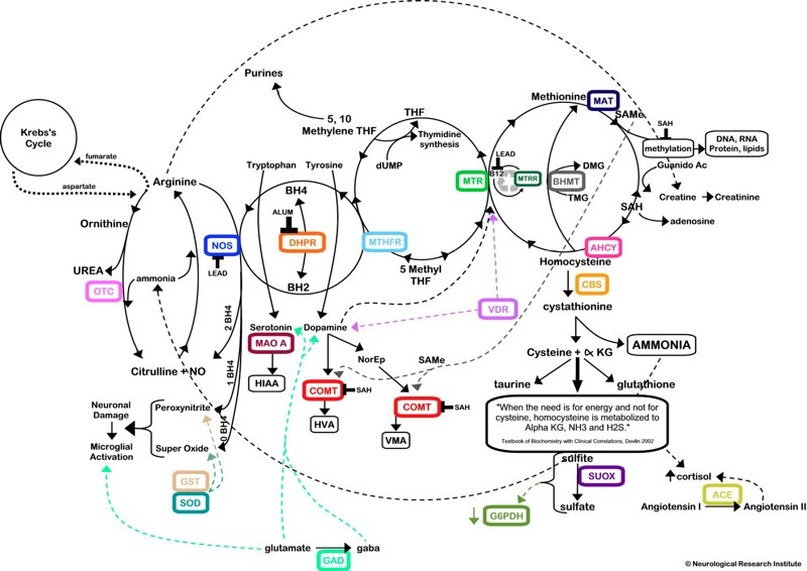
In Figure “a” below, we see a typical description of what is called the methionine cycle. It is my own version (I originally published these diagrams at the Annual Meeting of the Federation of American Societies for Experimental Biology [FASEB] in 2011.), but it typically shows how methionine is recycled, in order to conserve it maximally. I say typical, because this is the only metabolic picture generally shown in textbook descriptions of the methionine cycle. And it is accurate insofar as it applies when methionine levels are low, like when there is no methionine coming in from the diet, so methionine needs to be conserved.
 In this metabolic diagram, the green arrows refer to active metabolic pathways, and the dotted black arrows refer to inactive or minimally active pathways; the red, upper case abbreviations stand for enzymes (e.g., GNMT), whereas the names and abbreviations in black are metabolic intermediates (e.g., SAMe). The heavy red dotted line shows a metabolic brake or inhibition (i.e., an “off switch”) of GNMT by an intermediate called MeTHF. MeTHF is a form of folic acid which recharges the intermediate amino acid homocysteine (Hcy) by adding a methyl group to it, thus regenerating methionine. The presence of MeTHF is a signal that methionine is being regenerated because it is scarce, and it specifically turns off GNMT so that methionine is not wasted in this time of need. The SAMe that is generated is available for essential processes, such as the methylation of DNA bases, as shown by the green arrow across the top of the diagram.
In this metabolic diagram, the green arrows refer to active metabolic pathways, and the dotted black arrows refer to inactive or minimally active pathways; the red, upper case abbreviations stand for enzymes (e.g., GNMT), whereas the names and abbreviations in black are metabolic intermediates (e.g., SAMe). The heavy red dotted line shows a metabolic brake or inhibition (i.e., an “off switch”) of GNMT by an intermediate called MeTHF. MeTHF is a form of folic acid which recharges the intermediate amino acid homocysteine (Hcy) by adding a methyl group to it, thus regenerating methionine. The presence of MeTHF is a signal that methionine is being regenerated because it is scarce, and it specifically turns off GNMT so that methionine is not wasted in this time of need. The SAMe that is generated is available for essential processes, such as the methylation of DNA bases, as shown by the green arrow across the top of the diagram.
But what is not generally appreciated by biochemists and nutritionists is that when methionine is abundant, especially when methionine is being absorbed after a high-protein meal, the liver’s metabolic machinery switches gears (like it does in the transition after a high carb meal, when it switches from regenerating glucose to getting rid of glucose), working maximally to get rid of the excess methionine.
This situation is illustrated in Figure “b” below. In this diagram are also shown the two metabolic “on switches” (Solid yellow arrows with star points) for enzymes. Specifically, the high concentration of methionine itself cranks up the activity of MAT, which turns methionine into SAMe at a much higher rate. Most of this SAMe is not needed for essential methylation reactions, such as making DNA bases, but is instead deliberately wasted by GNMT, which has been turned on by the release of the braking action of MeTHF (shown in figure “a”). That’s because MeTHF is no longer being made, because SAMe itself shuts off MTHFR, the enzyme that makes MeTHF. Meanwhile, SAMe also turns on the enzyme CBS, which diverts homocysteine (“used” methionine) into the production of downstream sulfur-containing compounds (a pathway called “transulfuration”), including cysteine and glutathione, now that the remethylation (regeneration) of methionine has been turned off.
 Note especially that in this high-methionine condition, glycine is required both for the action of GNMT (a pathway called “transmethylation”) and for the transsulfuration pathway, to make glutathione. In this mode, glycine can be made both from serine and from scratch (i.e., from CO2 and ammonia), through the operation of a glycine-serine cycle. However, the liver cannot keep up with the need for glycine when methionine intake is too high relative to glycine intake (i.e., when we eat lots of muscle meat without the accompanying collagen from the bones and connective tissues), and glycine levels end up being inadequate to properly regulate the immune system. The result: chronic inappropriate and/or excessive inflammation. The antidote: Eat enough glycine to balance the high intake of methionine. 8 grams per day is about right for the typical omnivorous diet.
Note especially that in this high-methionine condition, glycine is required both for the action of GNMT (a pathway called “transmethylation”) and for the transsulfuration pathway, to make glutathione. In this mode, glycine can be made both from serine and from scratch (i.e., from CO2 and ammonia), through the operation of a glycine-serine cycle. However, the liver cannot keep up with the need for glycine when methionine intake is too high relative to glycine intake (i.e., when we eat lots of muscle meat without the accompanying collagen from the bones and connective tissues), and glycine levels end up being inadequate to properly regulate the immune system. The result: chronic inappropriate and/or excessive inflammation. The antidote: Eat enough glycine to balance the high intake of methionine. 8 grams per day is about right for the typical omnivorous diet.
http://180degreehealth.com/diet-inflammation-part-4/