The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43
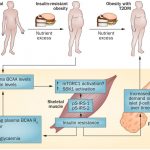
The gut microbiota affects nutrient acquisition and energy regulation of the host, and can influence the development of obesity, insulin resistance, and diabetes. During feeding, gut microbes produce short-chain fatty acids(SCFA), which are important energy sources for the host. Here we show that the short-chain fatty acid receptor GPR43 links the metabolic activity of the gut microbiota with host body energy homoeostasis. We demonstrate that GPR43-deficient mice are obese on a normal diet, whereas mice overexpressing GPR43 specifically in adipose tissue remain lean even when fed a high-fat diet. Raised under germ-free conditions or after treatment with antibiotics, both types of mice have a normal phenotype. We further show that short-chain fatty acid-mediated activation of GPR43 suppresses insulin signalling in adipocytes, which inhibits fat accumulation in adipose tissue and promotes the metabolism of unincorporated lipids and glucose in other tissues. These findings establish GPR43 as a sensor for excessive dietary energy, thereby controlling body energy utilization while maintaining metabolic homoeostasis.
Gpr43 was mainly expressed in the immune tissue and the white adipose tissue (WAT) of adult mice, as previously reported, but not in the brown adipose tissue (BAT). Gpr43 was abundantly expressed in the mature WAT but not in the BAT or in pre-adipocytes.
In this study, we showed that SCFAs activate GPR43 to regulate the energy uptake in WAT, which leads to the regulation of energy expenditure in other tissues, including liver and muscle. We also reported that GPR41, another SCFA receptor, regulates body energy expenditure by activating sympathetic nervous system at the level of ganglion. These findings indicate that SCFAs produced by gut microbiota, through activation of GPR41 and GPR43, have important roles in recognition of postprandial nutrient excess and orchestration of energy expenditure to maintain energy homoeostasis . In addition, the results obtained in our GPR43-overexpression mouse model indicate the possibility that overstimulation of GPR43 could be a strategy for therapy of metabolic disorders, including obesity and diabetes mellitus.
http://www.nature.com/ncomms/journal/v4/n5/full/ncomms2852.html