Amino Acid Racemization
Contents
Motion around a chiral center takes us from deep blue sea to deep dark space.
Racemization is the process in which one enantiomer of a compound, such as an L-amino acid, converts to the other enantiomer. The compound then alternates between each form while the ratio between the (+) and (–) groups approaches 1:1, at which point it becomes optically inactive.
Since (+) and (–) are experimental parameters, the absolute configuration of atoms in chiral molecules are commonly described using R and S, from the Latin rectus (right-handed) and sinistrus (left-handed). An older convention, commonly used by biochemists to describe amino acids and sugars, uses the letters D and L to designate absolute configuration (Figure 1). (See box, “Pasteur’s Racemic Revelation”.)
In a laboratory setting, scientists are able to measure the degree of racemization using polarimetry, liquid chromatography, capillary electrophoresis, and mass spectrometry. With these measurements, scientists can estimate the rate at which one enantiomer is converted to the other. Currently, these techniques are used to estimate the age of fossils, determine the life span of bowhead whales, and detect evidence of extraterrestrial life.
 |
| Figure 1. Hand It to Nature. While both left- and right-handed enantiomers (L and D, respectively) are available to nature, life is biased toward the use of L-amino acids and D-sugars. |
Chemical Whale Tales
Since 1981, whale hunters in Alaska have found at least six harpoon heads in bowhead whales that they have killed in the Beaufort Sea, southwest of the Arctic Ocean. The harpoon heads were made of stone or ivory. Anthropologists at the Smithsonian Institution (Washington, DC) estimate that they are between 130 and 200 years old and were probably used by Inupiat hunters in the late 1700s.
This circumstantial evidence led Craig George and fellow researchers at the Department of Wildlife Management (North Slope Borough, Barrow, AK) to believe that bowhead whales, named for their exaggerated jaw curve, could have a life span of more than 200 years. (To hear a radio interview with George, visit www.radio.cbc.ca/programs/quirks/archives/00-01/sep2300.htm.) The only other animals with similarly long life spans are the giant tortoise and the giant clam, which some have claimed can live about 150 and 220 years, respectively. They needed scientific data, however, to prove the whales’ longevity. Researchers turned to Jeffrey Bada and his colleagues at the Scripps Institution of Oceanography in La Jolla, CA, who are considered experts in the field of racemization analysis. By conducting aspartic acid racemization measurements using an HPLC/fluorescence detection method, Bada analyzed the bowhead’s eye lenses to estimate the whale’s age at the time of death (2).
The eye lens of a bowhead whale is made up of dozens of layers of protein, much like an onion. The first layer is completely formed while the animal is still in the womb, and then becomes biologically inactive (Figure 2). Following the onset of inactivity, racemization of amino acids begins and can be measured to estimate the amount of time since the initial deposit of the layer.
 |
| Figure 2. Biological Timeline. Similar to the rings on a tree trunk, the lens of a whale’s eye is composed of concentric spheres of protein, with the oldest rings nearest the center. By measuring the degree of aspartate race mization at the centermost sphere, researchers estimate the age of dead whales. |
Proteins are synthesized from L-amino acids. Once the L-form is incorporated, it begins to racemize. The longer the racemization process continues, the closer to 1 the ratio between the D- and L-forms becomes. This ratio allows chemists to approximate a birth date of a bowhead whale by estimating how long it has been since the first layer in the eye lens formed.Bada and his colleagues studied the eye lenses of 48 bowhead whale carcasses harvested between 1978 and 1996. The proteins in each lens nucleus were acid hydrolyzed into free amino acids, which were then derivatized. The levels of D- and L-aspartic acid were analyzed by ion exchange chromatography or reversed-phase HPLC. The relative proportions of each enantiomer were measured using fluorescence detection.
According to Bada, aspartic acid was used because it has the fastest racemization rate. “Unfortunately, none of the proteins’ amino acids racemize as fast and thus, there is, in general, no detectable amounts of racemization other than for aspartic acid,” he explains.
Following this analysis, Bada turned over the results of the chromatography measurements to Judith Zeh, a statistician at the University of Washington in Seattle, to estimate the ages of the bowhead whales. Using a racemization rate of 1.18 × 10–3/year, she calculated that four whales were more than 100 years old, and one was estimated to be 211 years old.
Jurassic Racemization
In addition to studying animal life span, Bada also applied racemization analysis to the search for genetic material in ancient fossil and tissue samples. DNA stability is a major complication in the analysis of ancient genetic material. Additionally, characterization of nucleic acids can often be difficult and time-consuming.
Bada believes that the extent of amino acid racemization is a useful indicator of the extent of DNA degradation in ancient specimens (3). “Amino acid racemization is simply an indicator of the level of degradation of DNA. DNA degradation is difficult to quickly evaluate, and amino acid racemization is a proxy for this evaluation,” states Bada. By measuring the progression of amino acid racemization in these samples, researchers can assess whether nucleic acid analysis would be productive.
Depurination, a hydrolytic reaction, is the primary cause of nucleic acid degradation, and racemization is affected by some of the factors that influence this process. For example, at neutral pH, rates of aspartic acid racemization and DNA depurination are almost identical at temperatures from 45 to 120 °C.
Bada and his colleagues examined the degree of race mization of aspartic acid in archaeological and paleontological samples. According to their findings, no evidence of DNA could be found in samples in which the D/L ratio was higher than 0.08. Consequently, all samples with D/L ratios equal to or below 0.08 produced DNA sequences. For samples not embedded in amber, this translates to less than 50,000 years. This suggests that recovering DNA from dinosaur fossils, which are on the order of 10 million years old, is unlikely.Bada also pointed out that the racemization technique is especially useful because only a few milligrams of sample are needed for analysis, and results can be obtained in only a matter of days.
However, the degree of racemization, which has also been used to measure age, did not correlate well with values provided by other dating methods for these same samples. Also, D/L ratios measured for other amino acids showed little correlation with aspartic acid or with each other. These discrepancies could be the result of many environmental factors, such as water concentration, pH, ionic strength, and contact with catalytic surfaces.
In response, Bada explains that as long as the samples have not been contaminated by amino acids of recent origin, the relative racemization pattern should follow the order aspartate>alanine>leucine. “That is what makes the racemization studies so unique,” he says. “Deviations from the expected relative racemization pattern are a sure sign of the presence of contamination.”
Conclusion
The current techniques for synthesis, detection, and structure determination have enabled us to come a long way since Pasteur’s pioneering days. The past 150 years have witnessed the developments of NMR spectroscopy for analysis of molecular structure, myriad chiral chromatographic and electrophoretic techniques, and sensitive mass spectrometry techniques that are capable of distinguishing enantiomers based on fragmentation patterns.
The applications of such techniques range from the more down-to-earth estimation of bowhead whale age to the out-of-this-world search for extraterrestrial life. Stereochemistry applies to foods, odors, and drugs, and as such, the above techniques also apply to the separation, characterization, and synthesis of these compounds. “Chance favors the prepared mind,” Pasteur once said, and his statement still very much applies to new applications for chiral chemistry.
References
- Dubos, R. Pasteur and Modern Science; Science Tech Publishers: Madison, WI, 1998, p 13.
- George, J. C.; Bada, J.; Zeh, J.; Scott, L.; Brown, S. E.; O’Hara, T.; Suydam, R. Can. J. Zool. 1999, 77, 571–580.
- Poinar, H. N.; Hoss, M.; Bada, J. L.; Paabo, S.; Science 1996, 272, 864–866.
http://pubs.acs.org/subscribe/archive/tcaw/10/i02/html/02brignole.html
Racemization and isomerization of amino acids
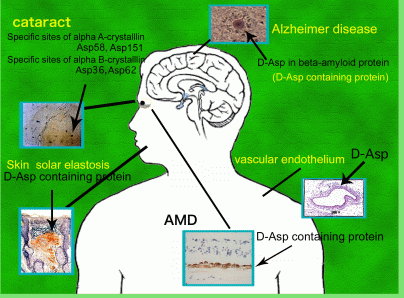
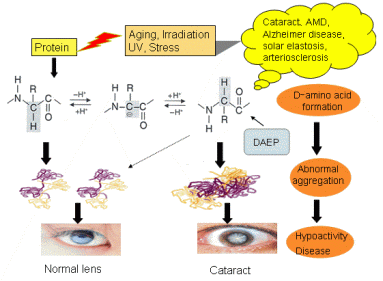
Protein consists exclusively of L- amino acids so as to form the proper folding structure and can function correctly in living body. This homochirality of amino acids was believed to be maintained throughout the entire lifespan. However, D-Asp residues, which are one of amino acids have been detected in various elderly human tissues such as lens of cataract, retina, conjunctivae and cornea of the age-related disease of eye, brain in Alzheimer disease, UV-damaged skin, teeth, bone, aorta in arteriosclerosis. We found that two specific aspartyl (Asp) residues in αA- and αB-crystallins, respectively, from human cataract lenses invert to the D-isomers to a high degree via a succinimide intermediate. This reaction proceeds by UV- (ultraviolet) and gamma-irradiation. D-Asp formation was accompanied by isomerization from the natural α−Asp to the abnormal β-Asp. Racemization and isomerization of amino acids in protein can cause major changes in protein structure, since different side chain orientations can induce an abnormal peptide backbone. Therefore, these posttranslational modifications can induce partial unfolding of protein leading to a disease state. In fact, α-crystallin containing large amounts of D-β-Asp undergoes abnormal aggregation to form massive and heterogeneous aggregates, leading to loss of its chaperone activity. We consider that the appearance of D-β-Asp in protein may the direct trigger of the change to the higher order structure and function. We are studying this theme by the following techniques; amino acid sequence analysis, mass analysis, 2D electrophoresis, CD analysis, analysis of optical isomers of amino acid by RP-HPLC, peptide mapping, western blotting.
The current research includes the following projects;
1) Study of racemization of amino acid residues in proteins and abnormal aggregation of proteins induced by aging, UV and gamma irradiation.
2) Study of mechanism of D- amino acid formation in protein.
3) Identification of D-β-Asp containing protein in UV-irradiated skin from elderly human donors.
4) Exploration of D-β-Asp containing proteins in age-related degenerated tissues.
5) Study of a specific enzyme that degrades D -Asp-containing protein.
6) Study of the radio-resistant mechanisms in the radio-resistant bacteria.
http://hlweb.rri.kyoto-u.ac.jp/fl/EN/english.html