Mechanisms of nanomaterials toxicity
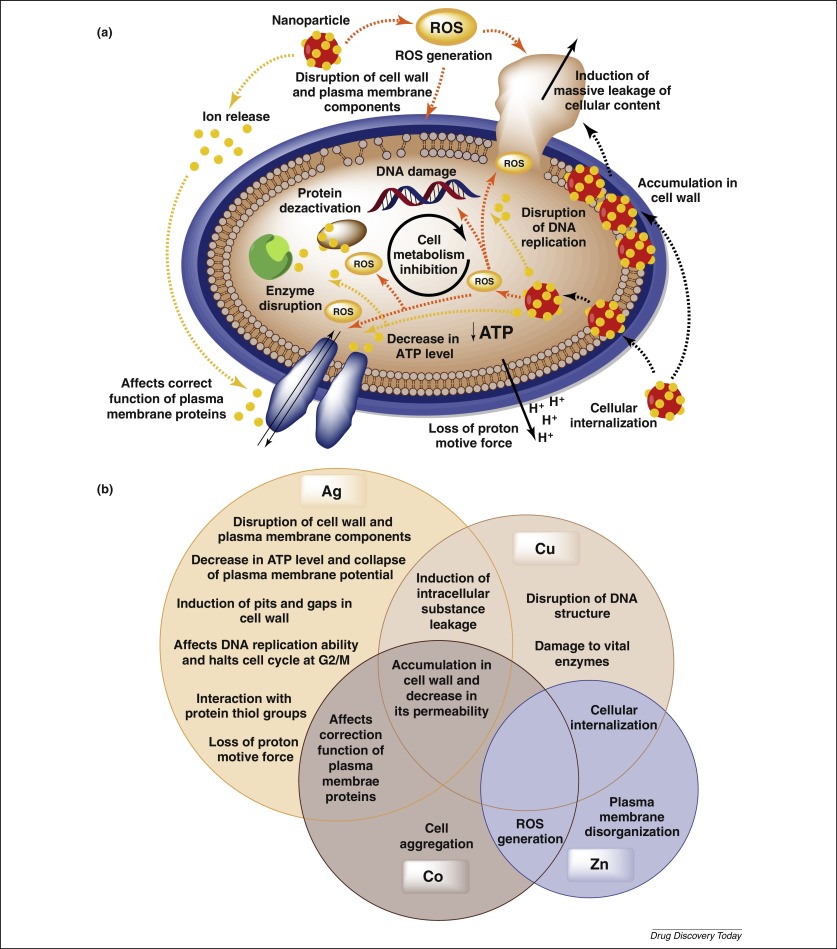
The most important mechanism of in vitro and in vivo nanotoxicity is related to the induction of oxidative stress by free radical formation, directly into the acidic environment of lysosomes [14]. Free radicals are released from phagocytic cells as a response to foreign material, insufficient amounts of anti-oxidants, presence of transition metals, or environmental factors [15]. The most exposed organs to nanomaterials are the liver and the spleen, especially due to the prevalence of phagocytic cells in their reticulo-endothelial system. In addition, the organs with high blood flow such as kidneys and lungs can be affected.
Hydrogen peroxide generated in vivo can react with silver nanoparticles and lead to releasing of Ag+ions [16]. In excess, free radicals cause the damage of cellular macromolecules through the oxidation of lipids, proteins, and DNA. Injury of cell membranes results in leakage of cytoplasmic contents and necrosis, whereas rupture of lysosomal membranes can induce apoptosis. In addition, the reactive oxygen species (ROS) can stimulate the inflammation through up-regulation of redox-sensitive transcription factors (nuclear factor kB – NF-kB, activator protein-1 – AP-1), and mitogen-activated protein kinases (MAPK) [15].
Intracellular, nanomaterials may interact with different components, especially with mitochondria and nucleus, considered as main sources of their toxicity. Nanomaterials such as metal nanoparticles, fullerenes, and carbon nanotubes are located in mitochondria and induce apoptosis and ROS formation [15]. The effect on nuclear DNA leads to nuclear damage, cell-cycle arrest, mutagenesis, and apoptosis. The action is due to the diffusion of nanomaterials into the nucleus and formation of ROS, which directly trigger DNA damage and induce chromosomal abnormalities [3]. Silver nanomaterials significantly up-regulate the gene encoding thiore-doxin-interacting protein (Txnip) and determine mitochondria-dependent apoptosis [17].
On the other hand, the oral silver administration did not induce micronucleus formation in rats following 28 days of treatment [18], suggesting lack of short-term toxicity.
It was demonstrated that silver nanoparticles were able to bind to thiol (-SH) groups within proteins and promoted their denaturation [19] including antioxidant enzymes such as superoxide dismutase and glutathione peroxidase. In blood, silver binds to albumin and decreases its ability to transport [20]. Gold nanoparticles can induce genotoxicity and block the transcription due to their ability to bind to DNA [21]. Some nanomaterials can stimulate the up-regulation of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) and xanthine oxidase in macrophages and neutrophils and generate free radicals. After absorption into the systemic circulation, the nanomaterials interact either with blood components and can lead to hemolysis and thrombosis or with the immune system and produce immunotoxicity.
Cho et al. [22] have shown that PEG-coated gold nanoparticles caused a transient inflammation and induced acute liver influx of neutrophils in parallel with increase of adhesion molecule levels (ICAM-1, E-selectin, and VCAM-1), chemokines, and inflammatory cytokines (IL-1β, IL-6, IL-10, IL-12β, and TNF-α) in liver up to 24 hours. Moreover, Lanone et al. suggested that intermediate metabolites of nanomaterials generated in the liver can contribute to hepatotoxicity [6]. Abdelhalim and Jarrar have shown that gold nanoparticles (10, 20, and 50 nm) administered for 3 or 7 days induced alterations in the hepatocytes, portal triads, and sinusoids. The changes were size-dependent and related with time exposure of nanoparticles and were explained by interaction with proteins, hepatic enzymes, and local antioxidant defense system [23].
Uptake of Ag nanoparticles in mammals
At present, there are few references related to the bioavailability of Ag NPs to terrestrial animals. Most of the information comes from laboratory studies using highly controlled exposure methods. From these studies, and the few that have looked at environmentally relevant exposures, it is possible to determine a rough picture of the bioavailability of Ag NPs to terrestrial organisms. However, many questions remain essentially unknown, especially with respect to realistic exposure models.
Once terrestrial animals come into contact with Ag NPs, the physiological route of exposure also needs to be considered as each pathway will confer different bioavailability on the host. Uptake of Ag NPs to terrestrial organisms can occur via air, soil and water, and these uptake routes all confer potentially different bioavailability. The primary route of uptake into terrestrial animals is likely to be via the lungs or through the gastrointestinal epithelia, with trans-dermal transport being a minor route. In addition, if retention of the NPs occurs in the animal, potential sources of transfer of NPs to offspring may occur through lactation or could be passed through the birth or the placenta. However, only very few studies have actually looked at bioavailability of Ag NPs to terrestrial animals with environmentally realistic exposure scenarios. One study has shown that once into contact with terrestrial organisms such as earthworms, Ag NPs were shown to bioaccumulate 5.1 ± 0.5% and 11.0 ± 0.3% of the Ag NPs and Ag+ (respectively) from the contents of their guts; after 48 h, the corresponding values were 0.38 ± 0.03% and 2.3 ± 0.1%, Ag NPs and Ag+ respectively [152].
By far, the best sources of information regarding the bioavailability of Ag NPs comes from controlled laboratory studies, primarily performed on rats or mice and generally using either i.v., instillation or inhalation as means of controlled exposure. Some of these studies are summarized in review form by Kruszewski et al. [153]. The bioavailability of orally administered Ag NPs was 1.2% in rats treated with 1 mg kg−1 Ag NPs and 4.2% in animals treated with 10 mg kg−1 Ag NPs [101]. Ag was found to be present in all examined organs of rats exposed to Ag NPs, with the highest levels in the liver and spleen for all Ag treatments [154]. In this study, Ag concentrations in the organs were highly correlated to the amount of Ag+ in the Ag NP suspension, indicating that mainly Ag+, and to a much lesser extent Ag NPs, passed the intestines in exposed rats [154]. In addition, it has been shown that Ag NPs injected in rats can be translocated from the blood to all the main organs and that the concentration of Ag in tissues was significantly higher in rats treated with 20 nm Ag NPs when compared with 200 nm Ag NPs [100]. The accumulation of Ag was also observed in all examined rabbit organs, including liver, kidney, spleen, lung, brain, testis and thymus up to 28 days following a single intravenous dose [155].
An uptake of Ag NPs into mammalian cells such as lung epithelial cells could be shown in vitro by TEM using an air–liquid exposure cell system to avoid particle dissolution (and aggregation) in cell culture media. The Ag NPs were endocytosed and highly aggregated inside vesicular structures, but they did not cause cytotoxicity or induce the release and expression of oxidative stress and (pro)-inflammatory markers. For equal AgNO3 exposure concentrations, similar effects were observed, and it has been concluded that no acute cytotoxic and (pro)-inflammatory effects for Ag at a realistic exposure dose can be assumed [156]. This is in agreement with another comprehensive review recently published by Chernousova & Epple [157], who showed that the risks for humans and the environment seem to be limited.
However, the fate of intracellular Ag NPs or Ag+ cannot be answered so far. The problem is that the majority of cell culture studies are done under suspension, and as already discussed it is then difficult to differentiate between the particle and ionic effects. One possibility to overcome the issue with suspension experiments is to use air–liquid exposure systems, if lung cells are considered in the study [156]. Using such sophisticated systems, the direct exposure of a defined solution with either Ag NPs or Ag+ onto the cell surface can be controlled, and therefore the possible observed effects can directly be linked to a particle or ion effect.
To the present, the only side effects to humans exposed to Ag+ are Ag deposits in colocalization with sulfur and selenium as found in patients with ‘argyria’ an irreversible discoloration of the skin [13,158]. However, the exact chemical reaction that leads to Ag deposition is still unclear.
http://rsif.royalsocietypublishing.org/content/10/87/20130396
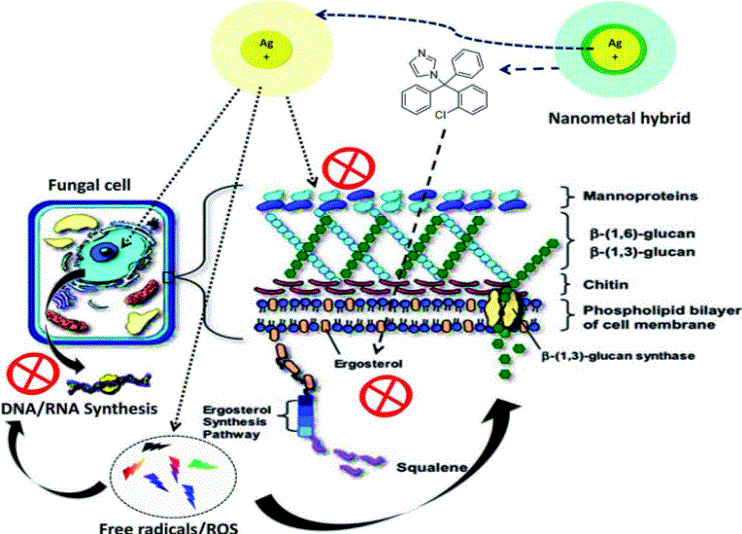
Nanotechnology unlocked distinctive platforms to move inside a hybrid therapeutic zone. Currently, nano-metal technology is the targeted field with exceptional advantages. Exceptionally small size and dominance of the surface properties such as high surface charge has raised a great deal of interest. This work is designed to exploit an interesting mechanistic feature i.e. multiple therapeutic targets possessed by metal nanoparticles. In this study we selected silver nanoparticles (AgNPs), which possess well documented anti-fungal activity and a standard antifungal molecule i.e. “clotrimazole”. A hybrid of AgNPs and clotrimazole was aimed to tackle clotrimazole resistance. Clotrimazole was firstly included into a β-cyclodextrin cavity to render it water soluble; subsequently the drug loaded dextrin moiety is functionalized on the surface of bovine serum albumin (BSA) stabilized silver nanoparticles using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysulfosuccinimide (EDC/NHS) chemistry. BSA stabilisation was essential to shield the physical interaction of AgNPs with the clotrimazole β-cyclodextrin complex that is otherwise experienced. Spectral and morphological characterization of the complex assures the synthesis of a hybrid metal complex. A cellular toxicity assay was performed to determine the toxic nature of the hybrid. This hybrid was then evaluated for its fungicidal activity on normal and clotrimazole resistant Candida cells. The toxicity and efficacy outcomes revealed a potent profile with a handy therapeutic window. Mechanistic explanations for this hybrid nature were supported by aggravated apoptotic cell percentage and reactive oxygen species production in both resistant and non-resistant cells. Cell cycle arrest studies further revealed G2/M phase cell cycle arrest, directing towards compromised cell membrane and DNA synthesis process equivalently in clotrimazole resistant cells.
http://pubs.rsc.org/-/content/articlelanding/2015/ra/c5ra08274a#!divAbstract